Warning: Undefined array key "hidden" in /home/clients/b69b93af3ae30d6d72cecf1c1e519fdd/web/wp-content/plugins/fusion-builder/shortcodes/fusion-gallery.php on line 756
Warning: Undefined array key "hidden" in /home/clients/b69b93af3ae30d6d72cecf1c1e519fdd/web/wp-content/plugins/fusion-builder/shortcodes/fusion-gallery.php on line 756
Warning: Undefined array key "hidden" in /home/clients/b69b93af3ae30d6d72cecf1c1e519fdd/web/wp-content/plugins/fusion-builder/shortcodes/fusion-gallery.php on line 756
Warning: Undefined array key "hidden" in /home/clients/b69b93af3ae30d6d72cecf1c1e519fdd/web/wp-content/plugins/fusion-builder/shortcodes/fusion-gallery.php on line 756
Warning: Undefined array key "hidden" in /home/clients/b69b93af3ae30d6d72cecf1c1e519fdd/web/wp-content/plugins/fusion-builder/shortcodes/fusion-gallery.php on line 756
Warning: Undefined array key "hidden" in /home/clients/b69b93af3ae30d6d72cecf1c1e519fdd/web/wp-content/plugins/fusion-builder/shortcodes/fusion-gallery.php on line 756
Warning: Undefined array key "hidden" in /home/clients/b69b93af3ae30d6d72cecf1c1e519fdd/web/wp-content/plugins/fusion-builder/shortcodes/fusion-gallery.php on line 756
The new generation of simple and effective blood test


An innovative molecular test
Colox® is a new generation blood-based test for early detection of
colorectal cancer. It accurately and reliably detects both adenomatous
polyps and early stages of colorectal cancer. It offers a convenient
solution for patients to be tested with no need for bowel or stool
preparation. It can be ordered by the physician as part of a routine
medical check-up.
Based on the principle of host-tumor interaction
Colox® is a molecular test that measures the immune system response to colorectal lesions. The test is based on the analysis of peripheral blood mononuclear cells (PBMCs) isolated from a routine blood sample.
PBMCs are responsive to growing adenomatous polyps and colorectal carcinomas as part of the “host-response”. Colox® measures the combined response of gene expression profile of 29 biomarkers by real-time PCR in PBMCs and the concentration of 2 protein tumor markers in plasma. RNA markers, chanfed during the initial stages of the development of a lesion, allow for its early detection. The protein tumor markers ensure specificity.
The biomarker measurement data are interpreted by Novigenix’s proprietary algorithm which generates the Colox® test result. This algorithm is based on classifier combinations derived from state-of-the art mathematical analytical methods that underpin the reliability of the test.

Clinical validation and scientific data
A clinically validated test
Colox is clinically validated and currently available through clinical laboratories in Switzerland. Colox® has been validated through a multi-center clinical study in Switzerland including 782 people. Colox® is available as a laboratory-developed test and is highly adaptable to standard medical laboratory workflows. Colox® is also available as a CE-IVD kit.

Clinical validation study
The performance of Colox has been validated in a multicenter clinical study in Switzerland comprising 782 people :
Colox detected 78% of patients with colon cancer with a specificity of 92.2%.
Colox detected 52% of patients with adenomatous polyps with a specificity of 92.2%.
Less than 1 out of 10 people were tested positive when they did not have colon cancer or adenomatous polyps.
| Product | Sample | Sensitivity | Specificity |
|---|---|---|---|
| Adenomatous polyps > 1cm | |||
| Colox® | Blood | 52.3% | 92.2% |
| FIT (OC-sensor, 100ng/ml) | Stool | 23,7 – 27,9% | 94,4 – 97,0% |
| gFOBT (Hemoccult II) | Stool | 6,8% | 95,2% |
| Colorectal cancer (all stages) | |||
| Colox® | Blood | 78,1% | 92,2% |
| FIT (OC-sensor, 100ng/ml) | Stool | 69,2 – 75,0% | 93,4 – 95,0% |
| gFOBT (Hemoccult II) | Stool | 33% | 95,2% |
Observational study
In 2016, Novigenix launched PROSPEROS : a PROSPEctive and Retrospective Observational Study describing the post-market use and results of the colorectal cancer screening test Colox® in Swiss routine medical practice. It was conducted with 30 doctors and 432 patients. This study highlighted an increase in the detection of cancers and adenomas.
Discover our scientific publications
Indication and Interpretation

Indication
Colox® is intended for women and men with an average risk of colorectal cancer: aged 50 and over, no symptom of colorectal cancer, no history of cancer or hereditary syndromes or chronic inflammatory bowel disease.
Colox® does not require any special preparation. The test can be done at the same time as other blood tests during an annual medical check-up.

Results
Timeline
What you need to know about the Colox® test
> What is Colox®?
Colox® is an easy and efficient blood test for the detection of cancerous colorectal lesions. It also detects precancerous lesions such as adenomatous polyps.
Colox® is a screening test which identifies the high-risk cases of colorectal cancer and directs patients to a colonoscopy diagnosis when needed. It provides an additional screening tool for physicians and the population concerned in order to increase the rate of participation in screening and thus reduce the mortality rate of this cancer.
> What exactly does the test measure?
Colox® is based on the analysis of peripheral blood mononuclear cells (PBMCs) isolated from a routine blood sample. PBMCs are responsive to growing adenomatous polyps and colorectal carcinomas as part of the “host-response”.
Colox® measures the combined response of gene expression profile of 29 biomarkers by real-time PCR in PBMCs and the concentration of 2 protein tumor markers in plasma. RNA markers, chanfed during the initial stages of the development of a lesion, allow for its early detection. The protein tumor markers ensure specificity. These genes are involved in inflammation (mediators or regulators of inflammation), mobility, signals and cell proliferation.
> Does Colox® detect colic cancers of other kinds?
The Colox test has been characterized and validated on cases of colonic cancer of adeno-carcinoma type and on cases of advanced adenomas. The adenocarcinoma type represents 95% of colonic cancers. However, it has not been characterized and validated on other types of rare colic cancers (GIST, squamous…) The main international guidelines for colorectal cancer screening target the adenocarcinoma type as well as the main screening methods. (stool test, screening colonoscopy).
Learn more about the test : Development and Clinical Validation of a Blood Test Based on 29-Gene Expression for Early Detection of Colorectal Cancer
> How does it work ?
A tumor is able to send signals which will modulate the bone marrow cells differentiation mobilized in response to the tumor growth by generating pro-metastatic or pro-angiogenic cells for example.
Bone marrow myeloid cells are recruited to the tumor site to create an inflammatory microenvironment that promotes tumor cell proliferation, angiogenesis, and tumor escape to immune surveillance. These cells will migrate through the blood vessels to reach the tumor site. Circulating blood cells have gene expression profiles that can be used as indicators of the presence of a tumor. Thus, Colox® measures the immune response of host cells to signals indicating the presence of a tumor.
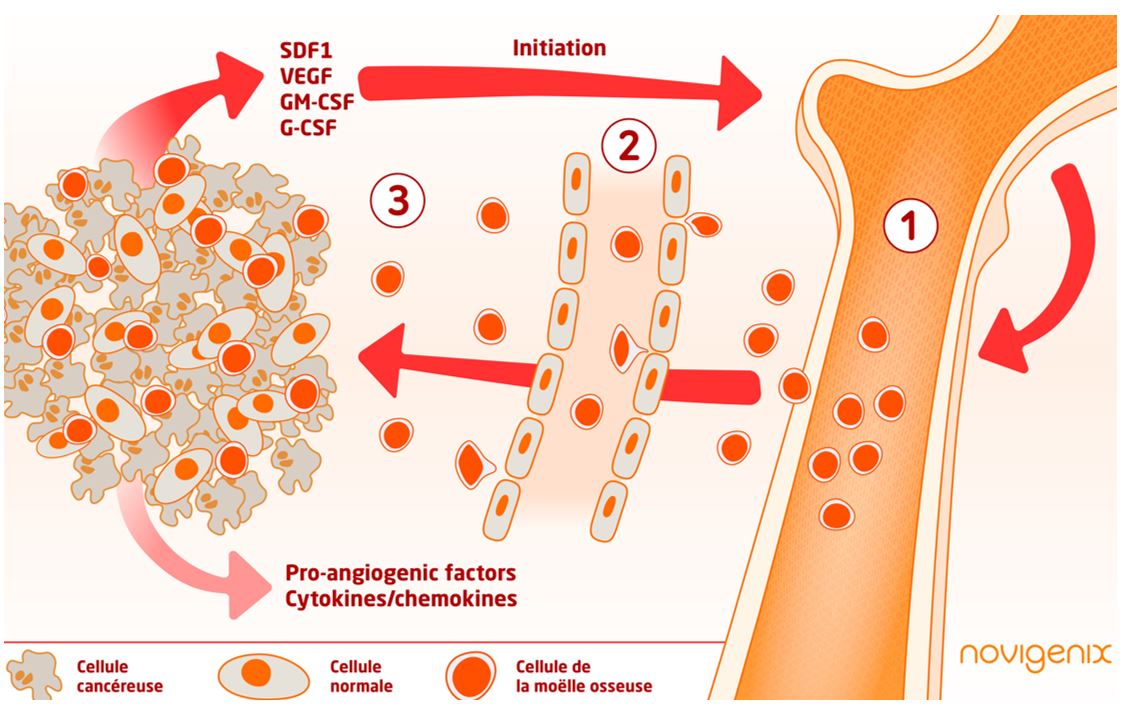
Instead of waiting for the tumor to release identifiable markers into the blood, Colox® relies on the very early response of the host to the tumor:
- Myeloid cells from the bone marrow are recruited by the tumor
- They will migrate to the tumor site through the blood vessels – It is during this step that PBMCs can be isolated and see if they have gene expression patterns characteristic of an immune answer to the tumor
- The cells arrive at the tumor lesion site and differentiate. An inflammatory microenvironment is created
Hypothetical scheme
> How is the test performed ?
Colox® is a molecular test which combines 29 RNA markers with 2 protein markers. A blood sample is taken from the patient and the PBMCs are separated. The RNA is extracted from the cells and then retro-transcribed into cDNA by RT PCR. The cDNA is then used for a quantitative PCR that will measure the expression level of these genes. The signal is analyzed using an algorithm. The Novigenix algorithm will generate a report that is then sent back to the lab. An abnormal level of expression of genes (overexpressed or over-expressed genes) gives a positive result.
> Is Colox® Reliable?
The performances of the test was validated in a multicenter clinical case-control study conducted in Switzerland, including 782 individuals. It detected 78% of patients with colon cancer and 52% of patients with adenomatous polyps. The test sensitivity for early stage detection is 61%. The specificity of the test is 92.2%, it means that less than 1 out of 10 people without any colorectal lesion tested positive.
Currently, 400 doctors have already prescribed Colox to more than 3,200 patients.
> What are the benefits of Colox® ?
Colox® is the most convenient screening test on the market that enables early diagnosis of colorectal cancer. It just needs a blood test compared to other more invasive and inconvenient tests, which require you to prepare your bowels or handle your stools. Moreover, it doesn’t require a hospital stay. Reliable clinical studies have proved the performance of the test for the effective detection of colorectal cancers. Moreover, Colox® has good performances for the detection of adenomatous polyps bigger than 1cm.
> Why a blood test ?
In Switzerland, only 10% of the target population agrees to perform a colonoscopy and 20% perform blood tests in the stool. This means that 70% of the population is not tested. A blood test has the potential to increase screening participation in the eligible population that is not currently screened. This test is therefore recommended for women and men who prefer the convenience of a blood test or who prefer the simplicity of a blood test to the invasive procedure of a screening colonoscopy.
> Who can do a Colox® test?
Colox® is a screening test for the prevention and the early detection of colorectal cancer. The test is intended for women and men with an average risk of colorectal cancer: aged 50 and over, no symptom of colorectal cancer, no history of cancer or hereditary syndromes or chronic inflammatory bowel disease.
> Who can’t do a Colox® test?
Colox® is not recommended for people who have a higher risk of colorectal cancer than average with:
- A personal history of adenomatous polyps or colorectal cancer
- A first-degree family history of colorectal cancer
- A family and/or personal history of a high-risk hereditary syndrome such as: Lynch syndrome (HNPCC), familial adenomatous polyps (FAP), etc
- A family and/or personal history of a high risk hereditary syndrome such as: Lynch syndrome (HNPCC), familial adenomatous polyps (FAP), etc
- A personal history of chronic inflammatory bowel disease (CIBD), Crohn’s disease, haemorrhagic recto colitis (HRC), etc
> Where can I find Colox® if I want to prescribe it?
Colox is available from our partner laboratories. You can call them to order the kit and take your patient’s blood sample directly to your office before 11am. A courier will pick it up. You can also prescribe the test and invite your patient to visit one of the partner laboratories to perform the test on site.
> Are there special recommendations before performing the Colox® test?
One of the advantages of Colox is that it does not require any special preparation. However, it is recommended you don’t smoke for 12 hours before the blood test. Some drugs can also influence the results. Ask your doctor for advice.
> How can I interpret the results?
- A negative test means that Colox did not detect any abnormality of markers associated to colorectal cancer and adenomas. The negative predictive value of the test is 99.9%. Periodic testing for colorectal cancer is recommended for the patient.
- A positive test does not necessarily mean that the patient has cancer. This means that Colox has detected variation in biomarkers associated to colorectal cancer or adenomatous polyps. The patient has adenomatous polyps with a probability of 52% (Positive predictive value) but only 2% of positive tests will be cancer. A positive result requires a follow-up diagnostic colonoscopy.
> What if a patient has symptoms while the test is negative?
Colox is an early detection test for colorectal cancer and colorectal lesions (adenomatous polyps). In case of symptoms, follow the medical guidelines recommended by the Swiss Society of Gastroenterology and the League Against Cancer Switzerland, which say to perform a colonoscopy. We remind you that a colonoscopy is the reference exam for people who have symptoms.
> How to explain a normal colonoscopy to a patient who has received a positive test?
The presence of a false-positive must not intervene in patient care and especially not in the suspicion of other gastrointestinal pathologies or presence of other adenocarcinomas. The fact that colonoscopy is negative is very reassuring because nowadays the rate of colorectal lesions missed at this examination is very low. Patients with a negative colonoscopy and a positive Colox test do not require further examination, but normal clinical follow-up is indicated. In this case it is likely a false positive of the Colox test.
> After how long do I have to repeat the test?
It is recommended to get screened every two years.
> How much the test cost?
Colox costs CHF 279. Colox is not reimbursed for the moment. Some insurances may cover a part of the cost, ask your insurance directly.
Colox® is currently available through our partners medical laboratories in Switzerland:
Labormedizinisches Zentrum Dr Risch
Waldeggstrasse 37, 3097 Liebefeld
Tel. 058 523 34 00
Fax.058 523 34 99
www.risch.ch
They believe in us

patients have already done the Colox® test

physicians have alrdeay prescribed the Colox® test
Colox is an alternative for individuals who absolutely don’t want to have a colonoscopy
For individuals without digestive symptoms and who absolutely don’t want to do a colonoscopy, two alternatives are proposed. One of them is the Colox® test. Contrary to colonoscopy, fecal occult blood testing and the Colox® test are non-invasive and less burdensome for individuals. In my experience, the Colox® test is probably much better than the colorectal test Hemoccult: there is no need to handle stool.
This new test has all the evidences of an effective and practical tool test for colorectal cancer screening
This new test has all the evidences of an effective and practical tool test for colorectal cancer screening in patients who should be referred for colonoscopy. This test is a promising help in cancer screening. Thanks to colorectal screening, we can prevent a lot of suffering. I advise to every men and women aged 50 and over to take colorectal cancer screening seriously and to get screened regularly.
Personally, I prescribe the colox test to patients from the age of 50
Colorectal cancer screening is an ongoing concern for practicing physicians. It is now possible to anticipate colorectal cancer thanks to a blood test. Personally, I prescribe the colox test to patients from the age of 50, during a check-up or during annual checks. I explain to them the purpose of this test which completes the clinical examination and the usual laboratory examinations. A negative test is reassuring for both the patient and the doctor and allows to giving up a screening colonoscopy. However, a positive colox test motivates the patient and the doctors to perform a colonoscopy.
Thanks to the positive result of Colox I did a colonoscopy which probably saved my life
Initially, I didn’t want to do a colonoscopy at all. My daughter advised me to do a Colox test so I finally decided to do it. The result was positive so I did a colonoscopy. During the examination, an abnormal polyp was detected and removed. I can testify that it is thanks to the positive result of Colox that I did a colonoscopy which probably saved my life. Over the paster 4 years, I am regularly checked and everything is in order.










